Objective: Thetherapeutic approach for transfusion-dependent non-severe aplastic anemia (TD-NSAA) is undetermined. Though the British guideline recommended intensive immunosuppressive therapy (IST) as front-line therapy for TD-NSAA ( Killick SB, et al. British Journal of Haematology. 2016; 172(2): 187-207), the cost and risk were relatively high. Hetrombopag is a thrombopoietin receptor agonist approved for IST-refractory severe aplastic anemia (SAA) by the China Food and Drug Administration in 2021 ( Peng G, et al. Ther Adv Hematol. 2022; 13: 20406207221085197). This study aimed to evaluate the efficacy of hetrombopag plus cyclosporine A (CsA) for TD-NSAA.
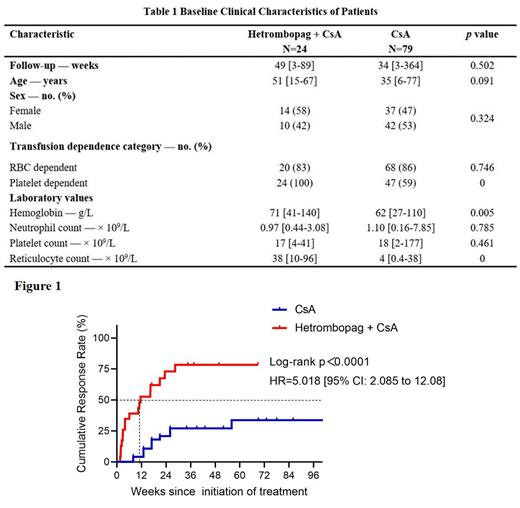
Methods: 24 eligible patients receiving hetrombopag plus CsA from September 2021 to July 2023 were collected by prospective registration (ChiCTR2100045895) in the Chinese Eastern Collaboration Group of Anemia (CECGA). A historical cohort (n=79) receiving CsA alone from December 2013 to January 2017 in the CECGA was used as a comparator (Table 1). Primary endpoint was overall response at 24 weeks, defined as hematologic response in ≥ 1 lineage. Secondary endpoints were overall response at 12 and 48 weeks, complete response at 48 weeks, overall survival, transformation to SAA and treatment-related adverse events.
Results: At 24 weeks, the overall response rate was 74% in hetrombopag plus CsA cohort and 16% in CsA cohort (odds ratio [OR]: 14.7; 95% confidence interval [CI]: 4.1 ~ 52.4; p<0.001). The overall response rates at 12 and 48 weeks were also significantly higher in hetrombopag plus CsA cohort than those in CsA cohort (43% vs 8%, OR: 9.1, 95% CI: 2.7 ~ 31.1, p<0.001; 85% vs 30%, OR: 13.0, 95% CI: 2.5 ~ 66.2, p = 0.001, respectively). At 48 weeks, the complete response rate was 23% in hetrombopag plus CsA cohort and 2% in CsA cohort (OR: 13.8; 95% CI: 1.3 ~ 146.8; p = 0.029). The median time to initial response was 11.6 [95% CI: 2.9~20.3] weeks in hetrombopag plus CsA cohort, significantly shorter than that in CsA cohort (hazard ratio: 5.0; 95% CI: 2.1 ~ 12.1; p<0.0001) (Figure 1). The median time to erythroid, platelet and neutrophil responses in hetrombopag plus CsA cohort were 20.6 [95% CI: 9.1 ~ 32.0], 16.4 [95% CI: 3.0 ~ 29.9] and 23.0 [95% CI: 10.6 ~ 35.4] weeks, respectively. The 1-year survival rates in hetrombopag plus CsA cohort and in CsA cohort were similar (90% vs 94%; hazard ratio: 1.9; 95% CI: 0.2 ~ 15; p = 0.5). The percentage of patients transforming to SAA was 8% in hetrombopag plus CsA cohort and 34% in CsA cohort (OR: 0.2; 95% CI: 0.0 ~ 0.8; p = 0.018). Treatment-related hepatic or renal injury occurred in 7 patients (29%) in hetrombopag plus CsA cohort with only one ≥ Grade 3; all gained remission after dose reduction or discontinuation. Multivariate analysis of clinical parameters of all patients from both cohorts revealed that adding hetrombopag to therapy was strongly correlated with response (OR: 43; 95% CI: 4 ~ 528; p = 0.003), as was lower lymphocyte count (OR: 0.5; 95% CI: 0.3 ~ 0.8; p = 0.011).
Conclusion: The combination of hetrombopag with CsA improved the rate, rapidity, and strength of hematologic response among patients with TD-NSAA, and lowered their risks of transforming to SAA.
Disclosure: This study is supported by National Natural Science Foundation of China (81900109). The authors declare that there is no conflict of interest.
Disclosures
No relevant conflicts of interest to declare.